Marble chips calcium carbonate caco 3 react with hydrochloric acid hcl to produce carbon dioxide gas.
Marble chips and nitric acid experiment.
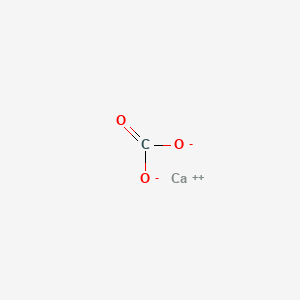
The chemical equation that is going to be followed throughout the experiment will be.
3m hydrochloric acid with large marble chips 1 5m hydrochloric acid with small marble chips 3m hydrochloric acid with small marble chips a reading was taken initially every 15 seconds for a minute and then every 30 seconds for the next 4 minutes.
Aim the experiment will be on the reaction of nitric acid and marble chips.
The rate of this reaction can be changed by changing the size of the marble chips.
An outline of an experiment that could be used to find the time and hence rate of reaction of marble chips and hydrochloric acid.
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
One for concentrated hno3 has no2 nasty nitrogen dioxide as a product while the other for dilute hno3 has not.
Using the apparatus shown the change in mass of carbon dioxide can be measure with time.
An investigation of the reaction between marble chips and hydrochloric acid.
Which statement about the second experiment is correct.
This was done as the first trial showed that in the first 2 minutes the.
The experiment is repeated using smaller marble chips.
Acid rain contains carbonic acid nitric acid and sulphuric acid co2 no2 and so2.
The reason i think it would vary is that you get different reactions when one puts pure copper in either concentrated or dilute nitric acid.
To investigate the effect of concentration on the reaction between marble chips and hydrochloric acid materials.
Calcium chloride solution is also formed.
The marble chip is calcium carbonate caco3.
Conical flask glass jam jars measuring cylinder stop clock watch with seconds stop watch app direct reading balance dilute hydrochloric acid cotton wool marble chips method.
All other conditions remain the same.
Marble is especially sensitive to the degrading by acidic chemicals also to weathering.
Place 40cm 3 of hydrochloric acid in an conical flask.
A the collisions are more frequent and higher energy.
Acid rain is one of the top degradation agents for marble artefacts around the world.